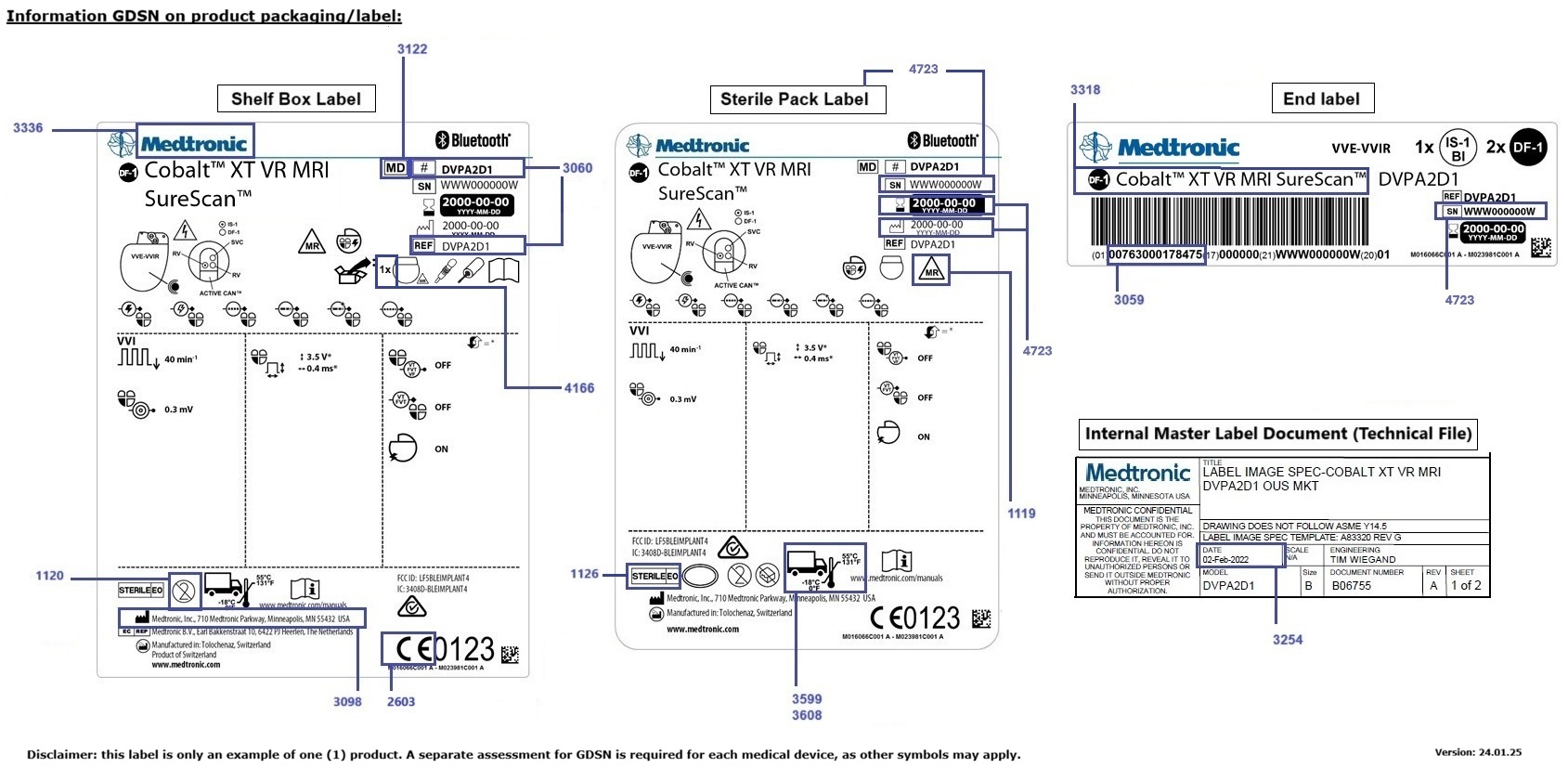
On this page, you will find the labels on the packaging of a product. The image shows the GS1 Data Source ID. The table below explains what it means.
|
|
GDSN information on product packaging / label |
|||
|
GS1 Data Source ID |
BMS ID |
Explanation Tip |
Used Symbol |
Meaning of symbol and (code to be entered) |
|
1120 |
1598 |
Manufacturer Declared Reusability Type Code |
|
SINGLE_USE |
|
1126 |
1593 |
Initial Manufacturer Sterilisation Code |
|
ETO_ETHYLENE_OXIDE |
|
|
GAMMA_RADIATION |
|||
|
|
DRY_HEAT |
|||
|
|
HYDROGEN_OXIDE |
|||
|
|
NOT_STERILISED |
|||
|
1127 |
1594 |
Initial Sterilisation Prior to Use Code |
|
Except for the code Not Sterilised (NOT_STERILISED) |
|
1119 |
1581 |
MRI Compatibility Code |
|
MRI_SAFE
MRI_COMPATIBLE
MRI_UNSAFE
Choose unspecified (UNSPECIFIED) if nothing is stated on the label |
|
4723 |
6364 |
UDI Production Identifier Type Code A batch number is indicated in the label as lot number
|
|
Lot or batch number (BATCH_NUMBER)
|
|
4723 |
6364 |
UDI Production Identifier Type Code |
|
SERIAL_NUMBER |
|
4723 |
6364 |
UDI Production Identifier Type Code |
|
MANUFACTURING_DATE
EXPIRATION_DATE |
|
2603 |
3070 |
Regulation Type Code Indication whether the product has a CE mark |
|
CE marking (CE) |
|
3059 |
67 |
GTIN (Global Trade Item Number) |
- |
N/A |
|
3060 |
68 + 69 |
Additional Trade Item Identification |
|
Reference number (MANUFACTURER_PART_NUMBER)
|
|
3074 |
66 |
Trade Item Unit Descriptor For example: box of 30 pieces |
- |
N/A |
|
3098 |
93 |
Manufacturer name |
|
Legal Manufacturer |
|
3122 |
161 |
Global Product Classification: GPC Brick Indication that the product is a medical device |
|
Medical Device (10005844) |
|
3254 |
144 |
Effective Date Time Effective Date Time concerns the date on which the specific version of the product information/label/packaging is valid |
- |
N/A |
|
3318 |
3517 |
Trade Item Description |
- |
Concerns the product description as it appears in the label of the packaging in the language or languages of the target market and any other languages used on the label. It is recommended to use brand name, function, net content, and, applicable at the box level, quantity of the next lower trade item. |
|
3336 |
3541 |
Brand Name |
- |
N.v.t. |
|
3599 |
3820+3821 |
Maximum Temperature and measurement unit |
|
Temperature limitations – maximum temperature in degrees Celsius |
|
3608 |
3826+3827 |
Minimum Temperature and measurement unit |
|
Temperature limitations – maximum temperature in degrees Celsius |
|
4166 |
1583 |
UDID Device Count. (sometimes indicated with a symbol) |
|
Number of items present in the base unit |
|
|
Other possible symbols on a packaging/label |
|||
|
993 |
1434 |
Does Trade Item Contain Latex |
|
Is there an indication on the item that the item contains latex (TRUE of FALSE) |
|
5317+5318 |
7237+7233 |
Claim Type Code and Element Claim Code |
|
Contains Phthalates (PHTHALATE) Mention a code from the ‘ClaimTypeCode’ codelist. If the item contains phthalates, select 'CONTAINS' for the 'Code type claim'. If the item does not contain phthalates, select 'FREE_FROM'. In case of doubt, select 'CONTAINS'.
|
|
4522 |
6089 |
Does Trade Item Contain Human Tissue |
|
Contains human blood or plasma Derivates.
Contains biological material of human origin. To be indicated with TRUE |
|
|
|
|
|
|